Understanding Canadian Health Care
Canada’s health care system is a publicly funded and provincially regulated, and universally accessible system designed to provide essential medical services to all Canadians. Here’s a brief overview of how cancer care is managed through Health Canada and the provincial health systems:
Health Canada:
- National Oversight: Health Canada is the federal department responsible for national health policies and regulations. It works to ensure that all Canadians have access to high-quality health care services, including cancer care.
- Funding and Standards: Health Canada provides funding to provincial and territorial governments and sets national standards for health care services. It also supports cancer research and provides guidance on treatment protocols and medications.
Provincial and Territorial Health Systems:
- Local Administration: Each province and territory is responsible for managing and delivering health care services to its residents. This means that cancer care can vary slightly depending on where you live, but all provinces and territories provide comprehensive cancer treatment and support.
- Cancer Care Services: Provincial health ministries oversee local cancer care facilities, including hospitals, cancer centers, and specialized clinics. They ensure that these facilities have the resources and expertise needed to provide effective treatment.
- Coordination and Access: Provinces and territories coordinate cancer care through regional cancer programs. These programs work to provide a range of services, including diagnosis, treatment, and follow-up care. They also facilitate access to specialists, such as oncologists and radiologists.
- Coverage and Costs: Cancer care, including consultations, treatments, and hospital stays, is typically covered under provincial health insurance plans. This means that most cancer-related services are provided at no direct cost to patients, though there may be some exceptions for specific types of care or additional services.
Patient Navigation and Support:
- Support Services: Many provinces offer support services to help patients navigate their cancer care journey. This can include patient navigators, counseling, and support groups to assist with emotional and practical challenges.
- Public Health Initiatives: Provincial and territorial health departments may also run public health initiatives aimed at increasing awareness about cancer prevention and early detection.
Canada’s health care system ensures that all residents have access to essential cancer care services. Health Canada provides national oversight and funding, while provincial and territorial health systems manage and deliver care locally. Through a combination of public funding, regional coordination, and support services, Canadians receive comprehensive cancer treatment and support throughout their journey.
Understanding Drug Approval in Canada
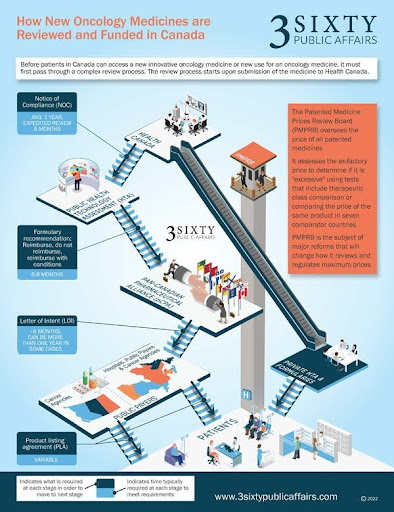
For many Canadians diagnosed with Inflammatory Breast Cancer, understanding how drugs are approved in our country is a common concern. In Canada, there is a systematic process carried out by several entities to determine and ensure the safety, efficacy, and prices of drugs before they are available to the public. It’s important to note that while Health Canada approves the drug for use, provincial systems play a crucial role in determining how and when these drugs are made available as part of publicly reimbursed treatment options. For a new cancer drug to progress from successful clinical trials to becoming a publicly reimbursed standard of care, multiple regulatory, evaluation, and funding steps are involved. We will outline them briefly here and then provide more detailed information on each step. See Figure 1 for a summary of the process.
Infographic source:
Gotfrit, J., Dempster, W., Chambers, J., & Wheatley-Price, P. (2022). The Pathway for New Cancer Drug Access in Canada. Current Oncology (Toronto, Ont.), 29(2), 455–464. https://doi.org/10.3390/curroncol29020041
The drug approval process
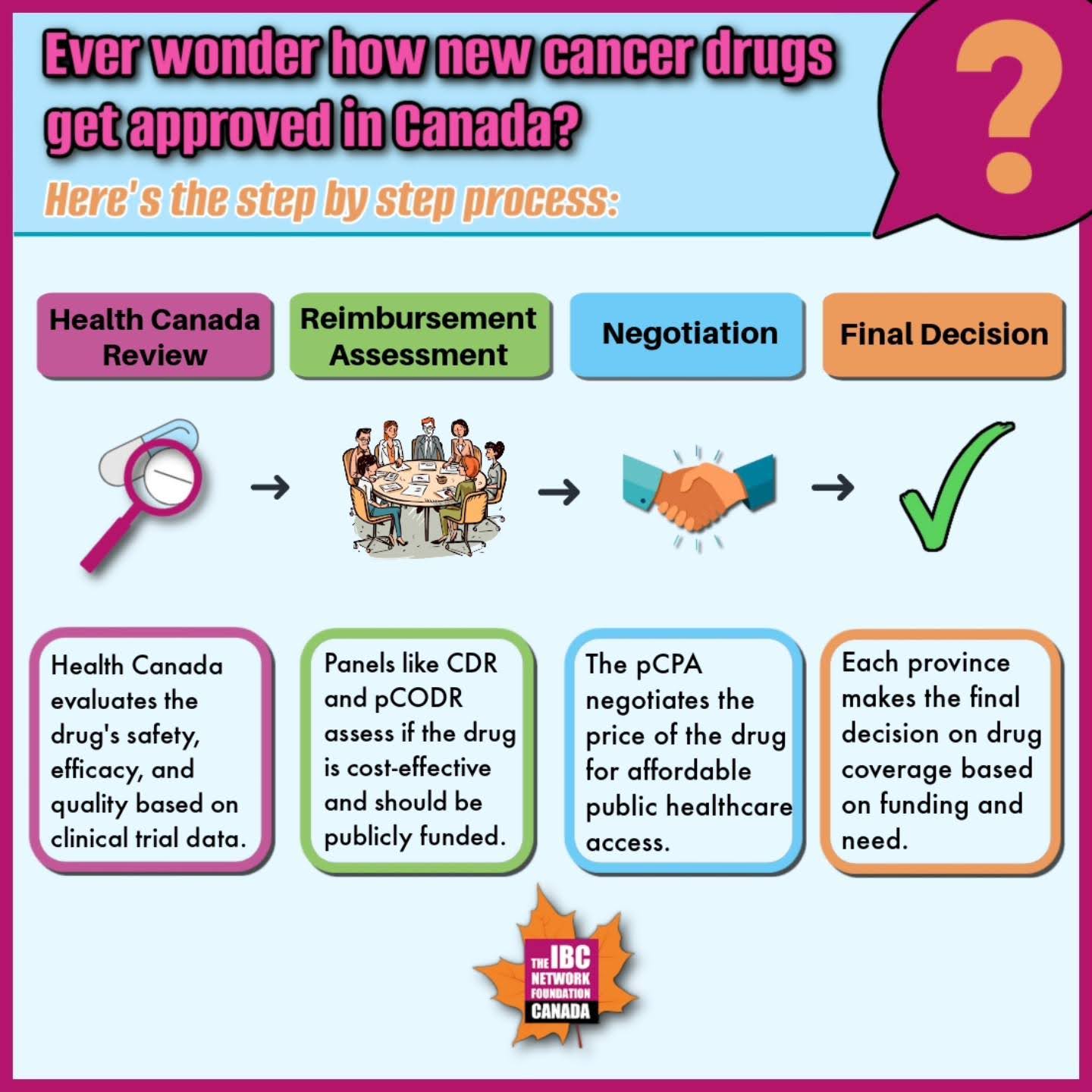
Health Canada Review and Approval
- The drug manufacturer submits a New Drug Submission (NDS) to Health Canada for review. This submission includes scientific data on the drug’s safety, efficacy, and quality, results of preclinical and clinical trials, therapeutic uses, and side effects.
- If Health Canada determines that the benefits outweigh the risks, it grants approval for the drug, issuing a Notice of Compliance (NOC) or Notice of Conditional Compliance (NOC/c) and a Drug Identification Number (DIN).
Assessment for Public Reimbursement
- The Common Drug Review (CDR) and pan-Canadian Oncology Drug Review (pCODR) assess the drug’s eligibility for public reimbursement. INESSS performs this role in Québec.
- Expert panels evaluate clinical evidence, cost-effectiveness, and patient perspectives, comparing the new drug with existing treatments.
Negotiation and Listing
- The pan-Canadian Pharmaceutical Alliance (pCPA) negotiates drug pricing on behalf of provincial, territorial, and federal drug programs.
- After negotiations, a letter of intent is signed, and each jurisdiction decides when to list the drug on their publicly funded formulary.
Final Decision
- The Executive Officer of drug programs at each provincial Ministry of Health makes the final decision based on committee recommendations, funding availability, and public interest.
Disparities in Access to Cancer Drugs Across Canada
Understanding the Issue
In Canada, access to cancer drugs varies significantly by province or territory due to the absence of a national drug insurance plan. Each region determines which drugs are covered, eligibility criteria, and out-of-pocket costs, leading to inconsistent access and financial burdens for patients.
Key Points to Know:
Coverage for Cancer Drugs
- IV Cancer Drugs: Generally covered when administered in hospitals or cancer centers, as required by the Canada Health Act.
- Take-Home Cancer Drugs (THCDs): Coverage for these oral medications varies widely. Provinces like Alberta, British Columbia, Saskatchewan, and Manitoba offer more comprehensive access. In contrast, Ontario and Atlantic provinces have fragmented coverage, often resulting in higher out-of-pocket expenses for patients.
Health Technology Assessment (HTA)
- Provinces independently assess new cancer drugs, leading to delays and inconsistencies. Even if a drug is recommended by national assessments, approval and coverage can differ by province, affecting drug availability.
Catastrophic Drug Coverage
- In some provinces, patients must incur substantial costs before qualifying for full coverage. For example, in Nova Scotia, a household earning CAD 65,000 may need to spend CAD 5,900 before full coverage applies. This financial burden can impact treatment adherence and outcomes.
Patient Support Programs (PSPs)
- Pharmaceutical companies may offer temporary financial aid through PSPs while a drug is pending approval or not covered by public insurance. However, these programs are often unreliable and not a long-term solution.
How This Affects IBC Patients
Inflammatory Breast Cancer (IBC) patients face significant challenges due to these disparities. Variations in drug availability and coverage can lead to delays in treatment and potentially poorer outcomes.
What Needs to Change
Adopt a Cancer Agency Model:
- Provinces like Ontario and those in the Atlantic region could benefit from a centralized cancer agency model. This approach would streamline drug access and reduce financial and administrative burdens, ensuring more equitable treatment across regions.
Create a National Drug Formulary:
- Implementing a pan-Canadian list of approved cancer drugs would standardize access to treatments, including THCDs, and address discrepancies between provinces.
What you can do
Stay informed about your province’s policies and advocate for improvements in drug access. Connect with patient advocacy groups to support efforts for policy reforms, including a national drug formulary and streamlined approval processes.
Helpful Resources
- How Drugs are Reviewed in Canada
- The Pathway for New Cancer Drug Access in Canada – PMC
- Disparities in Public Cancer Drug Funding across Canada
Addressing these access disparities is crucial for improving cancer care in Canada. By adopting streamlined models and national standards, policymakers can ensure equitable access to life-saving treatments for all Canadians.